Custom HCP ELISA
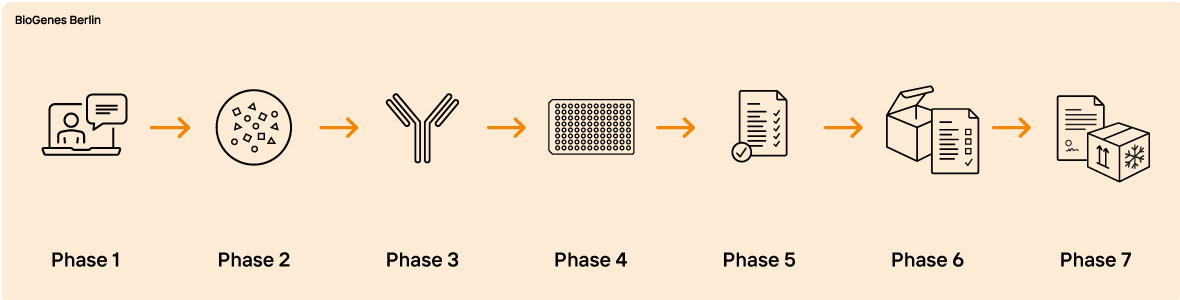
Are you looking for a reliable and customized HCP assay development process for your project? BioGenes has been developing process-specific HCP ELISAs since 1999. With over 400 projects for various cell lines, BioGenes has become a leading global specialist in this field. Our highly qualified scientists and a proactive project management team enable BioGenes to offer a complete portfolio of services. Our experience has resulted in a state-of-the-art specific HCP ELISA development approach that is appreciated by our customers worldwide.
It's important to note that the ELISA development process is time-consuming and typically takes between 1 to 1.5 years to complete. To ensure a smooth and efficient process, BioGenes recommends initiating your project at an early stage, preferably when the proof of concept has been achieved.