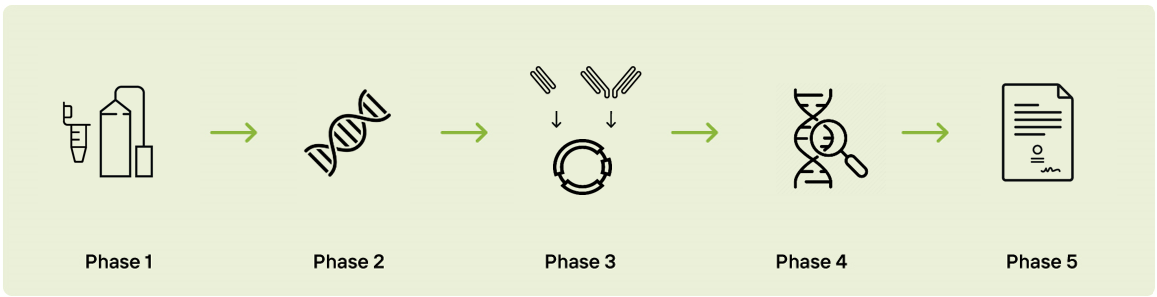
Phase 1 - RNA Isolation from Antibody Expressing Cells
For hybridoma clones stored at BioGenes, the clones will be recultivated and tested in an ELISA for activity. If the hybridoma sequencing is part of the “Take Care” or antibody production package, this work is already included. For foreign hybridoma clones, you have two options: you can either send us fresh cell pellets with 1x106 hybridoma cells (frozen, including one back-up vial), or you can send us two cryo vials of the same lot. Total RNA from 1x106 hybridoma cells will be extracted.