Phase 1 – Expert Consultation
- Definition of immunization strategy (protocol, species, animal number)
- Extensive discussion between BioGenes’ scientists, project manager and customer to provide a specific offer including defined work phases

In vitro diagnostics (IVD) examine samples such as tissue, blood or others to detect diseases and monitor health conditions. The most common IVD assays utilize technologies like PCR, ELISA, CLIA, lateral flow, and immunohistochemistry. These methods identify specific DNA/RNA segments or rely on specific antibody-antigen interactions to detect target components. A crucial element of the latter is the use of suitable antibodies. The sensitivity and specificity of these antibodies directly impacts the accuracy and reliability of the assays.
BioGenes specializes in producing high-quality antibodies in scalable amounts that meet the stringent requirements of IVD assays. This includes the development and production of both, monoclonal and polyclonal antibodies. Monoclonal antibodies are ideal for eliminating cross-reactivities due to their ability to specifically target a single epitope. On the other hand, polyclonal antibodies have the advantage of binding to multiple epitopes on an antigen, making them highly versatile for various applications.
BioGenes offers fully customizable immunization protocols for the generation of polyclonal antibodies. Clients can tailor these protocols to their specific needs, including the scheduling of boosts and bleedings, in consultation with our project management. Furthermore, we offer full flexibility to determine the immunization period and the number of animals involved. In addition to these tailored services, we ensure the superior quality of antibodies through affinity purification (total or specific IgG), making them highly reliable for IVD applications. Our strategy ensures consistent high quality antibodies throughout the life cycle of your IVD
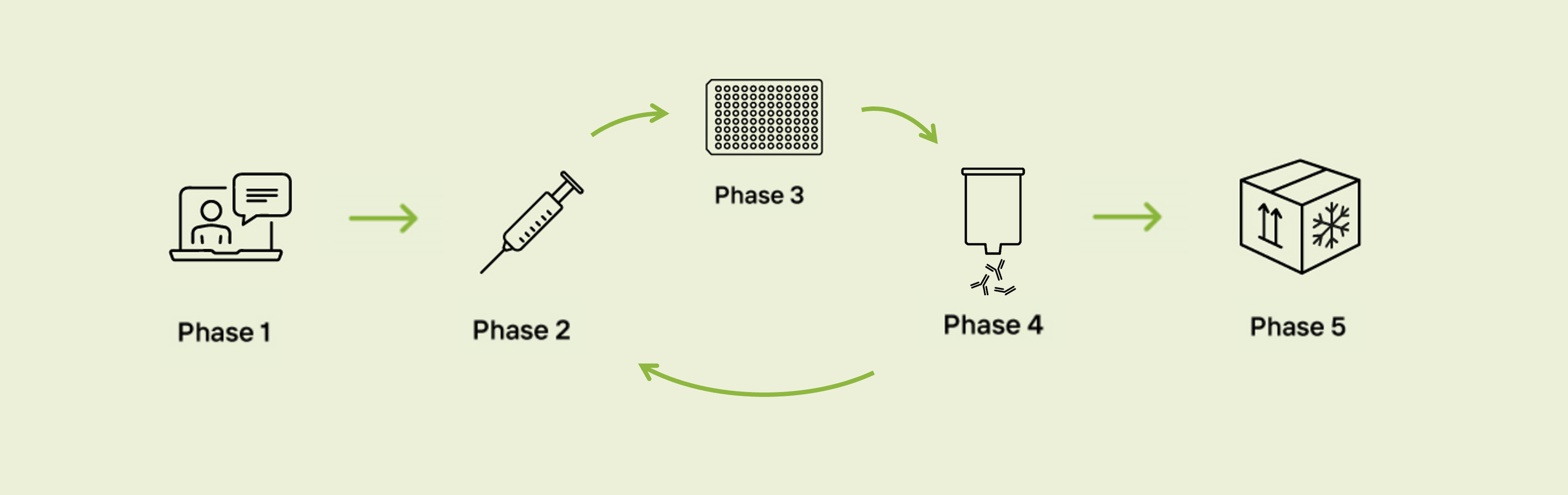
Phase 1 – Expert Consultation
Phase 2 – Immunization
Phase 3 – ELISA Titer Tests
opt. Phase 4 - Affinity Purification
Phase 5 – QC & Deliverables
Duration: dependent on the project goals and requirements
Contact
If you need more information, a specific offer
or want to talk to an expert
Which technologies are used as IVD Assays?